当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NiCrOx@silicalite-1 with Advanced CO2Utilization in Oxidative Dehydrogenation of Propane: Insights into Bifunctional Catalysis and Reaction Efficiency
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2025-06-04 , DOI: 10.1021/acssuschemeng.5c02033
Ruiqi Wu, Biaohua Chen, Ning Liu, Chengna Dai, Ruinian Xu, Gangqiang Yu, Ning Wang, Yubing Xu, Hongxia Han
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2025-06-04 , DOI: 10.1021/acssuschemeng.5c02033
Ruiqi Wu, Biaohua Chen, Ning Liu, Chengna Dai, Ruinian Xu, Gangqiang Yu, Ning Wang, Yubing Xu, Hongxia Han
|
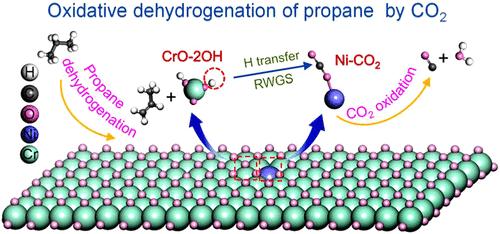
CO2-mediated oxidative dehydrogenation of propane (CO2-ODHP) has attracted great attention, as it not only efficiently favors propylene production but also provides a promising route for carbon neutralization. The present work has developed a highly efficient CO2-ODHP catalyst Ni2.1%Cr3.6%Ox@S1-1.0 with exceptional C3H8 conversion (51.7%), C3H6 selectivity (91.4%), long-term stability (passing through a 36 h test), and significantly enhanced CO2 conversion (21.9% → 52.7%). This can be closely related to the incorporation of Ni into the lattice of CrOx, forming NiCrOx, which facilitates the adsorption and activation of CO2, thereby promoting the timely removal of subtracted H from C3H8. Additionally, the evenly dispersed NiCrOx species encapsulated by silicalite-1 (S1) also play crucial roles in the remarkable reaction efficiency and stability of Ni2.1%Cr3.6%Ox@S1–1.0. The specific CO2-ODHP mechanism was systematically investigated based on the combined experimental (in situ FTIR and in situ UV–vis) and theoretical (Density Functional Theory) simulations, which illustrates a bifunctional catalysis process that the dehydrogenation of C3H8 to C3H6 predominantly occurs over the Cr site, while CO2 adsorption, activation, and subsequent reaction with dissociated H mainly occurs over the Ni site. The DFT-based microkinetic modeling quantitatively validates the significantly higher reaction efficiency following Ni incorporation (7.42 × 10–7 → 6.10 × 107 s–1), which is 14 orders of magnitude higher than that of the CrOx site. Electronic structure analyses further demonstrate that Ni incorporation efficiently reduces the band gap (2.43 → 0.95 eV) between the NiCrOx site and CO2, which is identified as the fundamental factor underlying the superior CO2-ODHP activity of Ni2.1%Cr3.6%Ox@S1–1.0. Generally, the present work has developed an efficient bifunctional catalyst for CO2-ODHP, which paves the way for other highly efficient catalyst designs.
中文翻译:

NiCrOx@silicalite-1 与高级 CO2 利用在丙烷氧化脱氢中的作用:双功能催化和反应效率的见解
CO2 介导的丙烷氧化脱氢 (CO 2-ODHP) 引起了极大的关注,因为它不仅有效地促进了丙烯的生产,而且还为碳中和提供了一条有前途的途径。目前的工作开发了一种高效的 CO 2-ODHP 催化剂 Ni2.1%Cr3.6%Ox@S1-1.0,具有出色的 C3H8 转化率 (51.7%)、C3H6 选择性 (91.4%)、长期稳定性(通过 36 小时测试)和显着增强的 CO2 转化率 (21.9% → 52.7%)。这可能与 Ni 掺入 CrOx 的晶格中,形成 NiCrOx 密切相关,这有利于 CO2 的吸附和活化,从而促进从 C3H8 中及时去除减去的 H。此外,硅石-1 (S1) 封装的均匀分散的 NiCrOx 物质在 Ni2.1%Cr3.6%Ox@S1–1.0 的显着反应效率和稳定性中也起着至关重要的作用。基于结合实验( 原位 FTIR 和原位 UV-vis)和理论(密度泛函理论)模拟,系统研究了特定的 CO2-ODHP 机制,这说明了 C3H8 到 C3H6 的脱氢主要发生在 Cr 位点上的双功能催化过程,而 CO2 吸附、活化和随后与解离的 H 的反应主要发生在 Ni 位点上。 基于 DFT 的微动力学模型定量验证了 Ni 掺入后反应效率显著提高 (7.42 × 10-7 → 6.10 × 107 s-1),比 CrOx 位点高 14 个数量级。电子结构分析进一步表明,Ni 掺入有效降低了 NiCrOx 位点与 CO2 之间的带隙(2.43 → 0.95 eV),这被认为是 Ni2.1%Cr3.6%Ox@S1–1.0 优异的 CO2-ODHP 活性的基本因素。总的来说,本研究已经开发了一种用于 CO 2-ODHP 的高效双功能催化剂,这为其他高效催化剂设计铺平了道路。
更新日期:2025-06-04
中文翻译:

NiCrOx@silicalite-1 与高级 CO2 利用在丙烷氧化脱氢中的作用:双功能催化和反应效率的见解
CO2 介导的丙烷氧化脱氢 (CO 2-ODHP) 引起了极大的关注,因为它不仅有效地促进了丙烯的生产,而且还为碳中和提供了一条有前途的途径。目前的工作开发了一种高效的 CO 2-ODHP 催化剂 Ni2.1%Cr3.6%Ox@S1-1.0,具有出色的 C3H8 转化率 (51.7%)、C3H6 选择性 (91.4%)、长期稳定性(通过 36 小时测试)和显着增强的 CO2 转化率 (21.9% → 52.7%)。这可能与 Ni 掺入 CrOx 的晶格中,形成 NiCrOx 密切相关,这有利于 CO2 的吸附和活化,从而促进从 C3H8 中及时去除减去的 H。此外,硅石-1 (S1) 封装的均匀分散的 NiCrOx 物质在 Ni2.1%Cr3.6%Ox@S1–1.0 的显着反应效率和稳定性中也起着至关重要的作用。基于结合实验( 原位 FTIR 和原位 UV-vis)和理论(密度泛函理论)模拟,系统研究了特定的 CO2-ODHP 机制,这说明了 C3H8 到 C3H6 的脱氢主要发生在 Cr 位点上的双功能催化过程,而 CO2 吸附、活化和随后与解离的 H 的反应主要发生在 Ni 位点上。 基于 DFT 的微动力学模型定量验证了 Ni 掺入后反应效率显著提高 (7.42 × 10-7 → 6.10 × 107 s-1),比 CrOx 位点高 14 个数量级。电子结构分析进一步表明,Ni 掺入有效降低了 NiCrOx 位点与 CO2 之间的带隙(2.43 → 0.95 eV),这被认为是 Ni2.1%Cr3.6%Ox@S1–1.0 优异的 CO2-ODHP 活性的基本因素。总的来说,本研究已经开发了一种用于 CO 2-ODHP 的高效双功能催化剂,这为其他高效催化剂设计铺平了道路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号