当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pKa Data-Driven Insights into Multiple Linear Regression Hydrolysis QSARs: Applicability to Perfluorinated Alkyl Esters
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2025-06-04 , DOI: 10.1021/acs.est.4c11596
Jovian Lazare, Caroline Tebes-Stevens, Eric J. Weber, Lindsay K. Shields
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2025-06-04 , DOI: 10.1021/acs.est.4c11596
Jovian Lazare, Caroline Tebes-Stevens, Eric J. Weber, Lindsay K. Shields
|
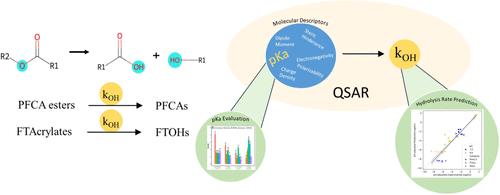
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are used in many applications due to their attractive nonstick and fire-fighting properties. When released into aquatic environments, commercial PFAS and associated byproducts may be transformed by hydrolysis to form more persistent products. For example, hydrolysis of perfluorinated carboxylic acid esters leads to perfluorinated carboxylic acids (PFCAs) and fluorotelomer alcohols (FTOHs). Quantitative structure activity relationships (QSARs) previously developed for predicting carboxylic acid ester hydrolysis rates are assessed for their predictive performance for perfluorinated molecules using compiled perfluorinated alkyl ester hydrolysis data. The assessment indicates that the model(s) are capable of estimating half-lives of various chain-lengths of perfluorinated alkyl esters; however, predictive performance could be improved through more accurate calculated chemical descriptor values for precursors of PFCAs. In particular, more accurate estimated pKa values are needed to improve hydrolysis rate predictions. The performance of several available cheminformatic applications (ChemAxon, SPARC, pkasolver, MolGpka and OPERA) is assessed for estimating pKa values for PFCAs and FTOHs, highlighting the need for improvements in predicting pKa values for longer-chain PFAS with perfluorinated α-carbons adjacent to the OC═O ester group.
中文翻译:

pKa 数据驱动的多元线性回归水解 QSAR 见解:对全氟烷基酯的适用性
全氟烷基和多氟烷基物质 (PFAS) 因其有吸引力的不粘和防火特性而被用于许多应用。当释放到水生环境中时,商业 PFAS 和相关副产品可能会通过水解转化为更持久的产品。例如,全氟羧酸酯的水解会产生全氟羧酸 (PFCA) 和含氟调聚物醇 (FTOH)。使用汇编的全氟烷基酯水解数据评估先前为预测羧酸酯水解速率而开发的定量结构活性关系 (QSARs) 对全氟分子的预测性能。评估表明,该模型能够估计全氟烷基酯的各种链长的半衰期;然而,通过更准确地计算 PFCA 前体的化学描述值,可以提高预测性能。特别是,需要更准确的估计 pKa 值来改进水解速率预测。评估了几种可用的化学信息学应用程序(ChemAxon、SPARC、pkasolver、MolGpka 和 OPERA)的性能,以估计 PFCA 和 FTOH 的 pKa 值,强调需要改进预测长链 PFAS 的 pKa 值,其中全氟 α-碳与 OC═O 酯基相邻。
更新日期:2025-06-04
中文翻译:

pKa 数据驱动的多元线性回归水解 QSAR 见解:对全氟烷基酯的适用性
全氟烷基和多氟烷基物质 (PFAS) 因其有吸引力的不粘和防火特性而被用于许多应用。当释放到水生环境中时,商业 PFAS 和相关副产品可能会通过水解转化为更持久的产品。例如,全氟羧酸酯的水解会产生全氟羧酸 (PFCA) 和含氟调聚物醇 (FTOH)。使用汇编的全氟烷基酯水解数据评估先前为预测羧酸酯水解速率而开发的定量结构活性关系 (QSARs) 对全氟分子的预测性能。评估表明,该模型能够估计全氟烷基酯的各种链长的半衰期;然而,通过更准确地计算 PFCA 前体的化学描述值,可以提高预测性能。特别是,需要更准确的估计 pKa 值来改进水解速率预测。评估了几种可用的化学信息学应用程序(ChemAxon、SPARC、pkasolver、MolGpka 和 OPERA)的性能,以估计 PFCA 和 FTOH 的 pKa 值,强调需要改进预测长链 PFAS 的 pKa 值,其中全氟 α-碳与 OC═O 酯基相邻。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号